Home › Overcoming the Obstacles of Paper-Based CSV: Digitizing Your Validation Process
The world of scientific research is advancing at an unprecedented pace, and with it, the need for efficient and accurate data management is becoming increasingly important. Paper-based CSV has been the go-to method for storing and managing research data for many years; however, more than this manual approach is needed as technology evolves. The limitations of paper-based CSV are numerous. Firstly, it is prone to human error, and researchers often need to correct it when transcribing data from paper to computer. Secondly, it is time-consuming, with researchers spending hours manually inputting data. Finally, it is difficult to store and share, and large amounts of paper take up valuable space, making collaboration between researchers difficult.
Fortunately, many digital tools help researchers manage their data more efficiently. These tools are designed to automate the data management process, reducing the risk of human error and saving valuable time. They also allow for easy storage and data sharing, making collaboration between researchers more accessible. As the amount of data generated by scientific research continues to grow, it is clear that more than paper-based CSV is required. By embracing digital tools, researchers can ensure that their data is accurate, efficient, and easy to manage. It will improve the quality of scientific research and help advance our understanding of the world around us. Continue reading to discover how digital computer system validation tools can safeguard your research and reputation when it truly counts.
Paper-based CSV is the traditional method many industries use to ensure their computer systems meet regulatory standards. It means providing the digital tools and systems used for experiments, data analysis, and research to comply with necessary rules and work reliably. When relying on paper-based CSV, everything is documented manually. This method is often time-consuming and prone to errors. Think of it like writing down every step of an experiment by hand instead of using a computer to record it automatically.
This method has limitations and can sometimes take advantage of potential risks. Making it less effective in guaranteeing the accuracy and reliability of scientific data. In addition, the regulations guiding paper-based CSV are stringent. They demand correct documentation, validation, and maintenance of paper systems. It ensures adherence to set standards for data safety, accuracy, and traceability. It’s important to note that paper-based CSV isn’t an inherently poor practice. With it, we have the valuable groundwork for data analysis and replication; however, in today’s landscape, embracing more advanced validation management can significantly enhance:
Paper-based CSVs can pose various ethical challenges due to their inherent limitations and potential risks. Transcribing data manually can lead to errors. They are potentially compromising the accuracy and integrity of the data. It raises many ethical concerns, especially in clinical studies where accuracy is crucial for scientific validity. Paper-based CSV sometimes needs more security measures to protect sensitive information. Unauthorized access to physical documents or loss of papers can result in confidentiality and privacy breaches. It raises both ethical and legal concerns.
Conversely, paper-based CSV records may not be easily accessible to all authorized personnel involved in the study. This limitation could hinder timely decision-making and data analysis. They are impacting the overall efficacy of the study. In addition, maintaining a comprehensive audit trail becomes a lot more challenging with paper records. It makes meeting regulatory standards and compliance requirements even more difficult. The lack of traceability can hinder the ability to track changes, which is critical for ensuring accountability and transparency in research.
Moreover, ensuring the long-term preservation of valuable research data poses a huge concern. Paper documents are more susceptible to physical damage, deterioration, or loss over time. It raises questions about the reliability and reproducibility of study findings. To tackle these ethical hurdles, shifting to digital systems for collecting, organizing, and storing data can help reduce the risks linked to paper-based CSV. These digital systems provide better security, accessibility, traceability, and sustainability, thus boosting ethical standards in clinical research.
Digital solutions streamline the validation process by automating tasks previously done manually. For scientists, this means spending less time documenting and more time focusing on their research. Imagine using software that automatically records experimental data or analyzes results. This efficiency can accelerate scientific progress significantly. Digital CSV offers better risk management capabilities compared to traditional paper-based approaches. Scientists can identify and address potential risks more effectively through digital tools that provide real-time tracking, monitoring, and analysis. It helps ensure the reliability and validity of research data.
The scientific community is increasingly recognizing the advantages of digital transformation. Government agencies are also pushing for digital tools as they make it easier to mitigate risks and meet strict standards. They further align with the scientific community’s pursuit of accuracy, reproducibility, and integrity in research. The use of digital CSV brings exciting opportunities for scientific innovation. It combines advanced tech such as machine learning, AI, and extensive data analysis. It empowers scientists to push boundaries and gain deeper insights from their research. The shift from paper-based CSV to digital solutions in scientific research is about more than embracing modern technology. It’s all about giving scientists more power, reducing risks, following rules, and creating an innovative culture to drive progress in the field.
In CSV, taking a risk-based approach means evaluating possible risks and organizing validation tasks based on their importance. This method zeroes in on spotting and dealing with risks that affect how dependable, accurate, or compliant the computer systems used in scientific research are. This method highlights putting resources where they’re most needed depending on how risky certain system parts, actions, or features are.
A risk-based approach directly tackles the limitations of paper-based CSV by shifting the focus from exhaustive documentation to targeted risk assessment. Rather than evenly addressing every aspect, a risk-based approach lets scientists focus on the most crucial system parts or functions. This way, they can optimize their validation efforts and resources. Various methodologies and frameworks facilitate the implementation of a risk-based approach in CSV. These include:
Each framework offers structured approaches for examining, ranking, and managing risks in computer systems, ensuring that systems are reliable and compliant. By adopting a risk-based approach, scientists can focus on the most critical aspects, making the validation process in scientific research significantly more efficient. This reiteration of the method’s efficiency and reliability serves as a strong encouragement for scientists to embrace the risk-based approach in their CSV practices.
Transitioning to a digital computer system validation (CSV) approach involves several vital parts that significantly enhance the research process. Here are some features to look for when exploring digital solutions.
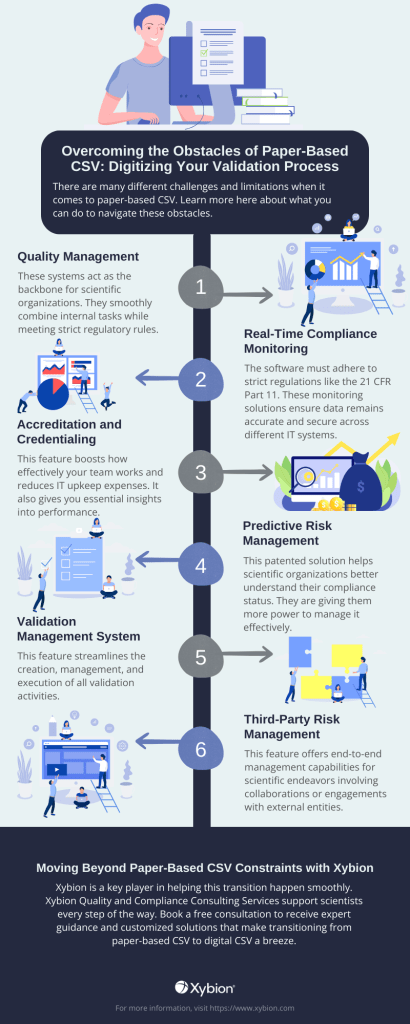
These systems act as the backbone for scientific organizations. They smoothly combine internal tasks while meeting strict regulatory rules (like those outlined by the FDA). They also help team up with outside partners, suppliers, and regulatory authorities. Ensuring everyone works together smoothly for compliance and top-notch quality assurance.
The software must adhere to strict regulations like 21 CFR Part 11. These monitoring solutions ensure data remains accurate and secure across different IT systems, including file and database systems, laboratory tools, and manufacturing instruments. They provide real-time oversight crucial for maintaining compliance in scientific research environments.
This feature boosts the effectiveness of your team’s work and reduces IT upkeep expenses. It also gives you essential insights into performance, helping you use data to improve operations’ cost and efficiency.
This patented solution helps scientific organizations better understand their compliance status, giving more power to manage it effectively. Studying potential business risks and sorting out key actions can help boost team productivity and cut resource overuse. This approach ensures a proactive stance toward managing compliance.
Predicting compliance risk requires specialized algorithms, machine learning models, or regulatory expertise that might only be found in some digital CSV handling tools; however, companies like Xybion provide all-in-one cloud platforms tailored for:
Equipped with real-time monitoring, Xybion platforms simplify your entire process by combining everything you need into a seamless platform.
Scientific research often spans multiple sites, countries, and regulatory guidelines. This feature streamlines the creation, management, and execution of all validation activities, ensuring a unified and standardized approach across diverse regulatory environments.
This feature provides comprehensive end-to-end management capabilities for scientific projects involving collaborations with external entities. It encompasses:
This approach ensures a thorough and efficient management of scientific endeavors.
Transitioning from paper-based computer system validation (CSV) to digital solutions in scientific research comes with challenges. Some research professionals may be concerned about the reliability and security of digital systems (compared to familiar paper-based methods). Addressing these concerns through comprehensive training, robust data encryption, and demonstrating the reliability of digital systems can alleviate apprehensions. Guiding teams through best practices for using digital solutions is crucial. Providing clear protocols, training sessions, and support during the transition helps them adapt smoothly to new technologies while maintaining compliance with regulations.
Be sure to highlight the long-term advantages of digital CSV. While the initial transition might pose challenges, emphasizing the efficiency gains, enhanced risk management, and potential for scientific innovation can motivate others to embrace the change. Offering strategies to mitigate short-term challenges is essential. It includes providing technical support, troubleshooting resources, and creating feedback loops to tackle immediate problems scientists might encounter during the transition. Remember to maintain ongoing support post-transition. Creating a responsive support system and providing access to resources for skill development ensures scientists feel supported and confident in using digital CSV tools effectively.
Adopting digital computer system validation (CSV) processes signifies a departure from the limitations and challenges of traditional paper-based validation. This shift to digital solutions revolutionizes the research landscape by providing scientists with tools that streamline workflows, enhance security, and facilitate real-time monitoring and analysis. This transformation bolsters research integrity and accelerates the pace of scientific innovation.
As businesses and organizations evolve, embracing new technologies that can help streamline processes and increase efficiencies is essential. One area ripe for innovation is scientific research, which traditionally relies heavily on paper-based documentation and validation processes. Xybion offers a solution to help organizations move beyond these constraints and into a more digital and automated future. By implementing Xybion’s advanced computer system validation (CSV) solutions, businesses can streamline their processes and reduce the risk of errors and compliance issues. Xybion’s comprehensive solutions are designed to meet the unique needs of companies in various industries, including life sciences, pharmaceuticals, medical devices, and more. Xybion is a key player in helping this transition happen smoothly. Xybion Quality and Compliance Consulting Services support scientists every step of the way. Book a free consultation to receive expert guidance and customized solutions that make transitioning from paper-based CSV to digital CSV seamless and straightforward.
We use cookies to improve your experience. By continuing to use our site, you accept our use of cookies. Privacy Policy and Terms of Use.
Cookie SettingsAccept