Computer System Validation
Computer systems validation, testing and compliance services
Computer systems validation, testing and compliance services
Home › Consulting Services for Regulated Industries | Xybion › Computer System Validation
If you are seeking computer system validation experts, look no further. Xybion has demonstrated expertise validating computer programs for all types of regulated businesses in the energy, transportation, financial services, and life sciences (including FDA-regulated businesses such as pharmaceutical and medical device manufacturers, clinical research organizations, and GLP laboratories).
We offer a global experience to oversee GxP or other compliance standard needs from our headquarters in the United States or offshore in our Global Delivery Center in Chennai, India.
Xybion Validation Specialists have successfully completed over 1,000 validation projects. Are you ready to be next?
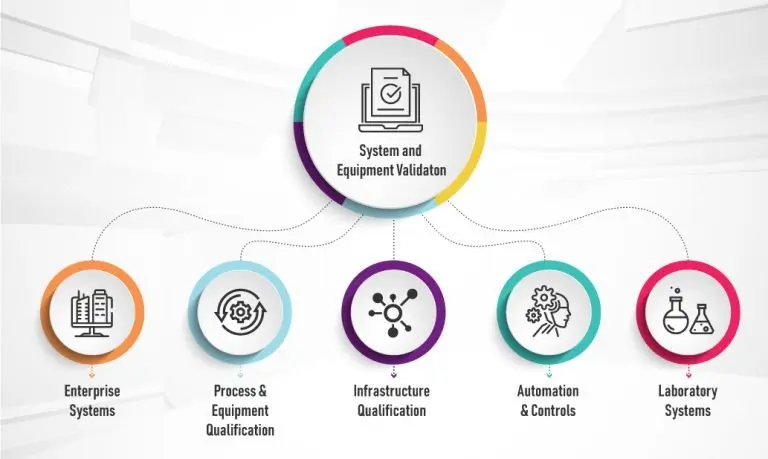
Xybion experts have a thorough understanding of federal regulations and industry-accepted software development standards. Our clientele is comprised of industries regulated by the FDA, FCC, IRS, and SEC. We utilize a five-step process for all computer system validation projects:
Enterprise Systems including CRM, ERP, EAM and SFA
Laboratory Systems including Laboratory Operations (R&D, QA/QC)
Quality Systems including CAPA, Data Management, Clinical Trial Applications, and SharePoint
Pharmacovigilance and Medical Information Systems
We use cookies to improve your experience. By continuing to use our site, you accept our use of cookies. Privacy Policy and Terms of Use.
Cookie SettingsAccept