
Overcoming the Obstacles of Paper-Based CSV: Digitizing Your Validation Process
There are many different challenges and limitations when it comes to paper-based CSV. Learn more about what you can do to navigate these obstacles here.
Regulated industry insights




There are many different challenges and limitations when it comes to paper-based CSV. Learn more about what you can do to navigate these obstacles here.
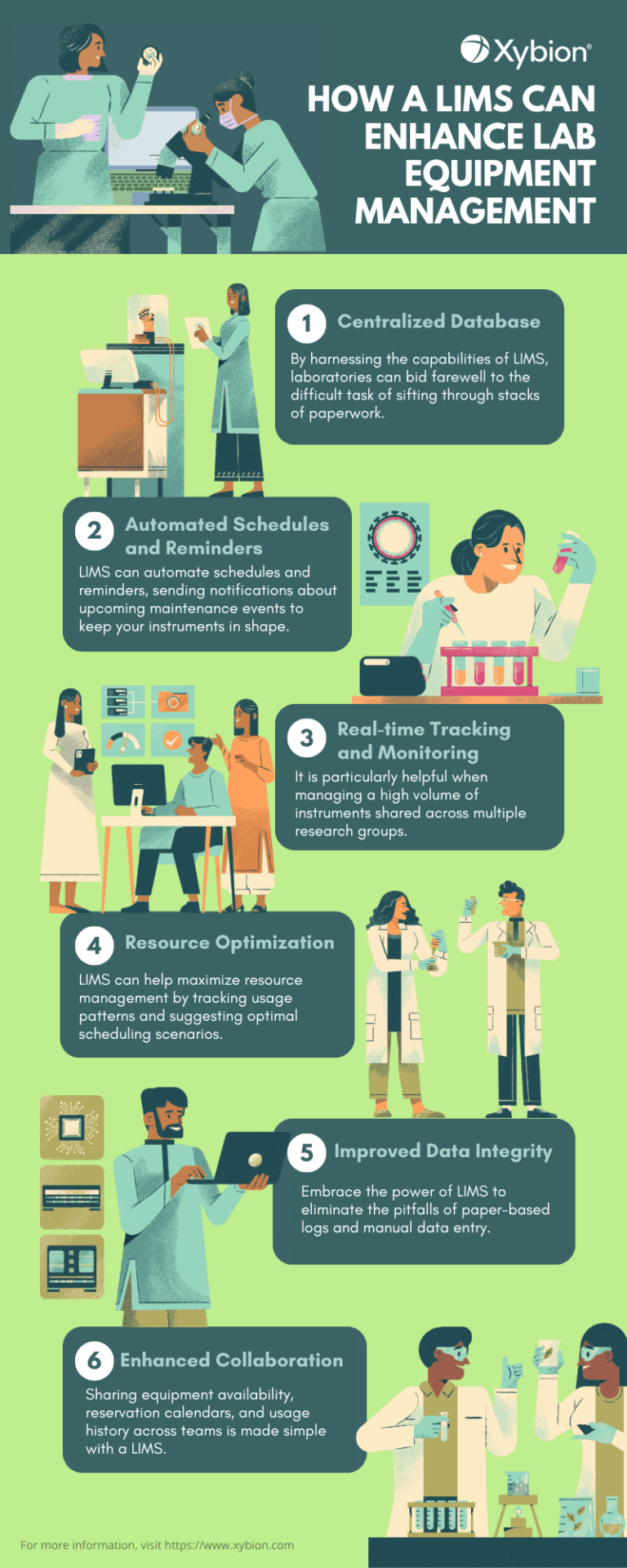
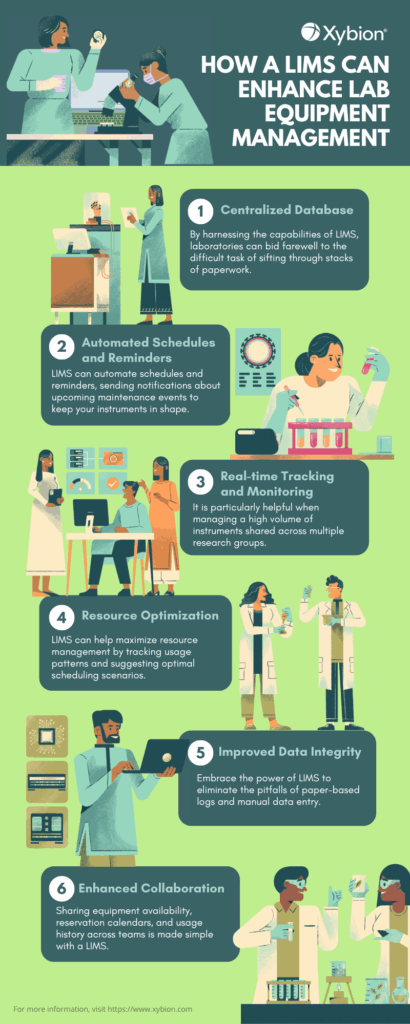
Explore the impact of LIMS on lab equipment management. Let us help you enhance the overall efficiency of your laboratory. Learn more.
Explore the important role of electronic data capture (EDC) systems in preclinicalstudies to comprehensively understand their modern research methodologies.

Mastering CDISC SEND standards doesn’t have to be complicated. By reading this guide, learn how to improve CDISC SEND standards and ensure compliance.

Developing a successful new drug application (NDA) requires careful planning and execution. Learn more on how to increase your chances of success.

LIMS is critical for protecting your laboratory’s sensitive data. Read and learn more about the role and benefits of LIMS security in your laboratory.
We use cookies to improve your experience. By continuing to use our site, you accept our use of cookies. Privacy Policy and Terms of Use.
Cookie SettingsAccept