Quality Assurance and Quality Control for Biotech Industries
Discover essential strategies for optimizing quality assurance and control in biotech industries. Ensure FDA compliance and improve product safety today.
Discover essential strategies for optimizing quality assurance and control in biotech industries. Ensure FDA compliance and improve product safety today.
Home › Quality Assurance and Quality Control for Biotech Industries
Did you know that the global biotech industry is expected to be worth more than $3 trillion by 2030? With a number like that, it isn’t surprising that biotechnology is gathering so much attention. With the ability to affect most areas of our lives, this could soon be the most important technology sector.
But in an arena as vast as biotechnology, how do you maintain quality? Projects require multiple professionals, often working in different locations, as well as advanced technology platforms, all working together. Read on to learn about the importance of quality assurance and quality control within the biotech industry.
Regulatory compliance remains a constant challenge for biotech companies, particularly with shifting global regulations. A well-integrated QMS is an essential tool for simplifying compliance by offering built-in reporting features and ensuring documentation is accurate, complete, and easily accessible during audits. Additionally, it optimizes processes such as batch record reviews, non-conformance reports, and CAPA investigations, reducing processing times and accelerating product release. With a QMS tailored to biotech needs, companies can effectively manage quality processes, minimize risks, and maintain compliance while driving innovation forward.
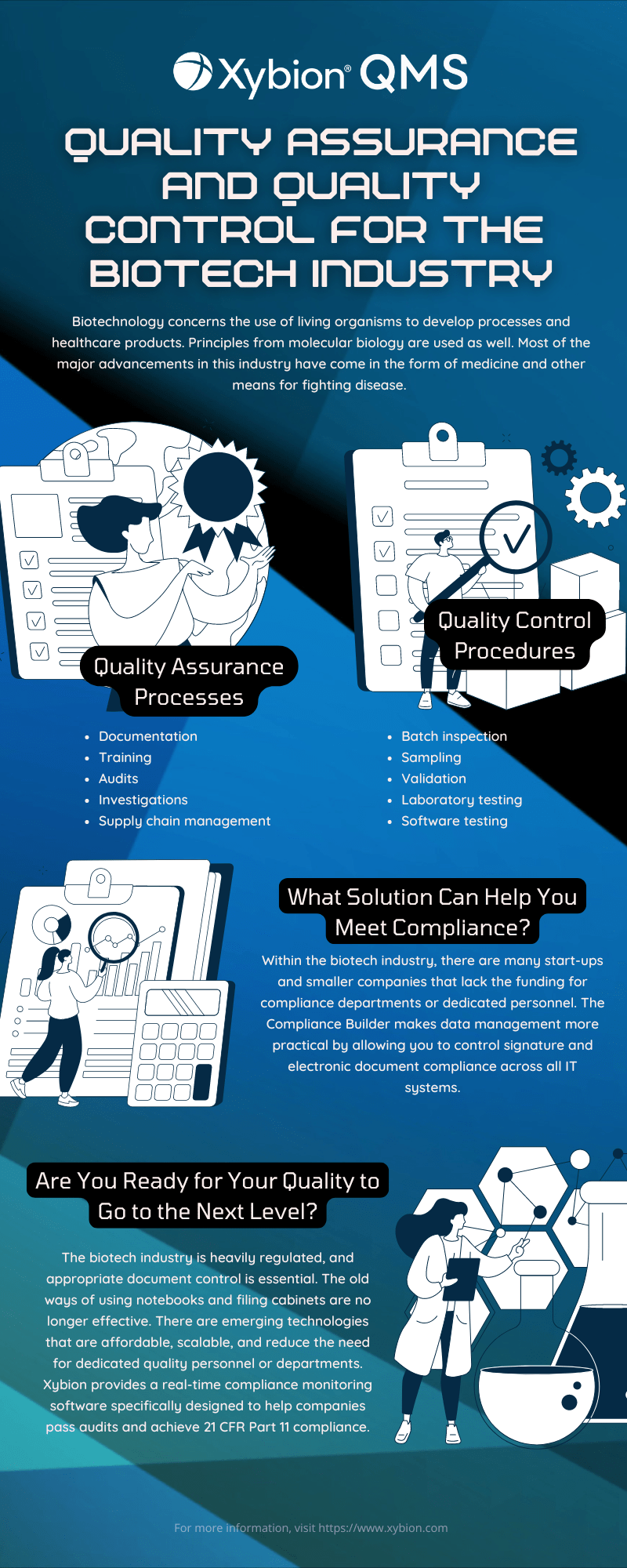
Biotechnology concerns the use of living organisms to develop processes and healthcare products. Principles from molecular biology are used as well. Most of the major advancements in this industry have come in the form of medicine and other means for fighting disease. Some of the other areas that biotechnology impacts include:
Biology isn’t the only science that this field relies on. It also uses concepts from mathematics, physics, and chemistry. Advanced technological applications like nanotechnology are also incorporated.
While they might sound like the same thing, quality assurance and quality control are different. Quality assurance includes all the steps that lead up to production. This is a verification that quality requirements are being met. It is a proactive process that aims to identify problems before they occur. It is systemic and focused on the creative process.
On the other hand, quality control is actually a subset of quality assurance. Quality control typically encompasses multiple inspections throughout the production phase. This is to ensure that the end product meets safety and quality standards. These inspections could just be simple documented observations or full tests.
This is a reactive process that finds faults with the finished product. It focuses on different parts and is all about verification.
Quality assurance is about prevention. It uses established processes that all personnel within the company are trained on. These processes include:
Quality control is results-oriented. It uses procedures to identify faults in the finished product. These procedures include:
The other major difference between these two quality realms is the personnel assigned to them. Quality assurance is more general and assigned to the whole team. From the time an individual is hired, some of their first days may include training in the quality assurance program.
As quality control is more specific, it has a dedicated personnel group. These individuals are trained in various forms of testing and can perform audits during and after the production process. Some large companies have entire departments dedicated to quality control.
Managing quality processes in the rapidly changing biotech industry is critical to ensuring product integrity and patient safety. Companies must comply with stringent regulatory standards, such as FDA guidelines, which require comprehensive documentation and rigorous oversight of manufacturing processes. A robust quality management system (QMS) helps streamline these tasks, enabling real-time tracking of deviations, corrective and preventive actions (CAPAs), and audit trails. By automating manual processes, a QMS significantly improves operational efficiency, ensuring quality standards are consistently maintained throughout the production lifecycle, fostering a sense of increased productivity.
Often, the products of the biotech industry are regulated by the U.S. Food and Drug Administration (FDA). The FDA has stringent requirements that require documentation at every step. This is required at every step, from conceptualization to the finished product.
Some of the major categories the FDA regulates include medical devices, vaccines, and cosmetics. The FDA has general regulations for all products that it oversees, but it even has specific regulations for items like animal studies.
This makes both quality control and assurance vital, not just in preventing errors but in meeting compliance. Both the processes of quality control and the testing of quality assurance must be documented.
Quality assurance and quality control have evolved with advances in technology. Prior methods of taking handwritten notes for documentation are inefficient and, in some cases, no longer meet current standards. Most large companies have moved into digital record-keeping.
The advent of cloud-based data storage has helped. Using the cloud assists with quality assurance and control by automating data backups and security updates, as well as ensuring overall security.
In today’s highly regulated biotech industry, maintaining a robust quality program isn’t just a best practice—it’s a necessity. Quality Assurance (QA) and Quality Control (QC) play critical roles in ensuring that your products and processes meet the required standards. As you’ve learned, understanding the differences between QA and QC can make all the difference in ensuring compliance, efficiency, and product excellence.
Relying on outdated methods for document control or compliance management is no longer viable. As regulations become stricter and the margin for error narrows, adopting advanced solutions is the key to maintaining compliance without overwhelming your resources. Xybion’s quality management software (QMS) offers the tools you need to stay ahead of regulatory requirements, reduce operational risk, and pass audits with ease. Now is the time to transition from manual processes to cutting-edge solutions. Xybion QMS is a scalable, affordable solution designed to help your organization achieve 21 CFR Part 11 compliance and meet the most stringent industry standards.
Book a demo of Xybion QMS today and discover how our powerful web-based software can enhance your compliance, improve efficiency, and ensure that your products consistently meet regulatory guidelines.
We use cookies to improve your experience. By continuing to use our site, you accept our use of cookies. Privacy Policy and Terms of Use.
Cookie SettingsAccept