Overcoming Challenges in Complying with SENDIG v3.1.1
Blog SENDIG v3.1.1 and the FDA Approval Process The cost of developing a new drug can be as high as $2.9 billion. Even after such
Regulated industry insights

Blog SENDIG v3.1.1 and the FDA Approval Process The cost of developing a new drug can be as high as $2.9 billion. Even after such

Blog The Connection Between Using a Document Management System and FDA Compliance Labs that receive FDA warning letters must waste valuable resources to become compliant
Blog Expansion of FDA Data Standardization Rules with FDAs DART Fit For Use Pilot In drug development, the Food and Drug Administration (FDA) issued final

Blog A Guide to the New Drug Application Process Since 1938, every new drug of a pharmaceutical company has been the subject of an approved

Blog The Right LIMS Solution for Your Lab The global market for LIMS software is expected to expand by a massive 7% by 2030 because
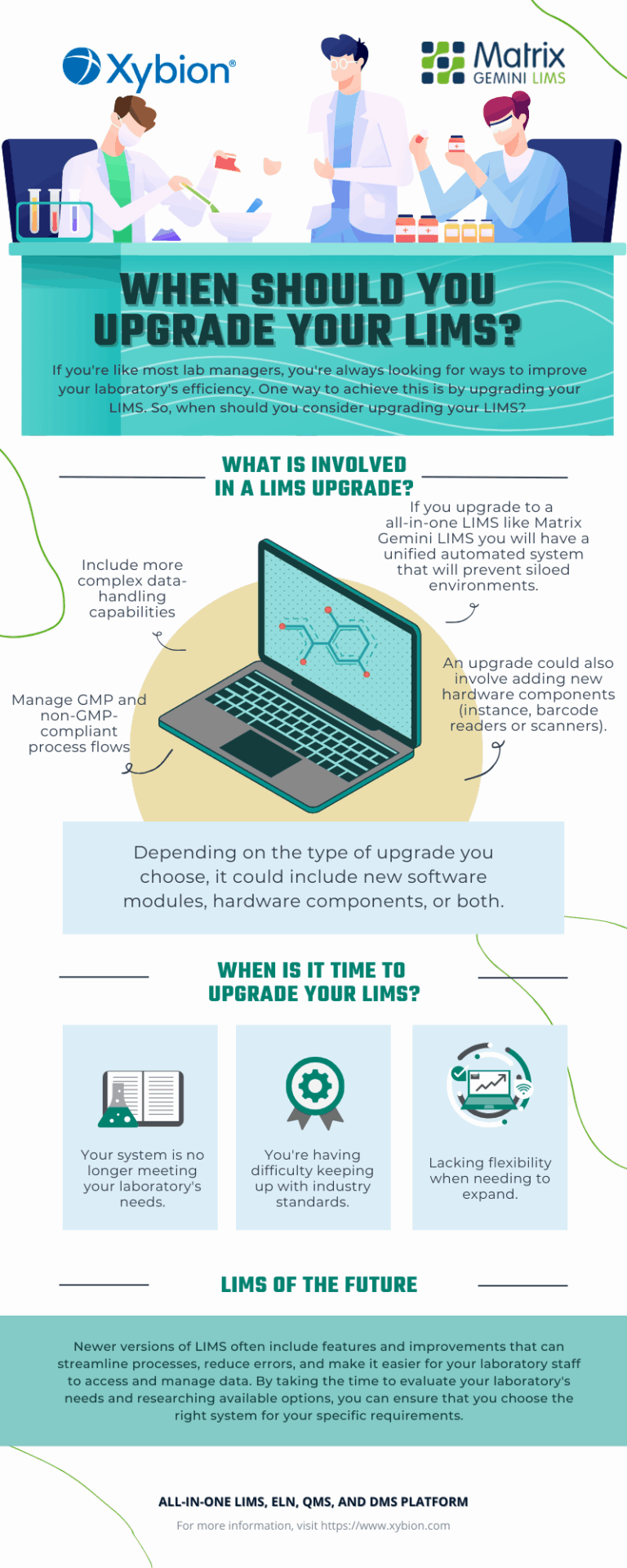
Blog The Right Time for a LIMS Upgrade In the US alone, over 60% of laboratories currently use a Laboratory Information Management System (LIMS) to manage their
We use cookies to improve your experience. By continuing to use our site, you accept our use of cookies. Privacy Policy and Terms of Use.
Cookie SettingsAccept